Chem.Eng.J. / Proportional electrochemical biosensor based on Cu (II) modified covalent organic skeleton for ultra-sensitive and specific detection of glutathione
backdrop
As we all know, due to the change of human life style and the rapid urbanization and industrialization, environmental pollution has seriously threatened our world.
Specifically, environmental pollutants are toxic, refractory and bioaccumulative, posing a high threat to public health and ecological security.
Human physiology consists of several complex reactions involving multiple biomolecules.
Even a small deviation can cause serious impediments to our normal functioning and thus prevent us from carrying out our daily activities with peace of mind.
Any deviation from homeostasis can usually be detected by changes in the concentration of certain biomolecules.
Researchers have developed various analytical techniques such as spectroscopy, chromatography, electrophoresis, and hyphenation techniques to detect environmental pollutants and biomolecules.
Although these methods are accurate and reliable, most of them have the disadvantages of complex instruments, time-consuming sample pretreatment, low sensitivity and high cost.
Compared with these methods, electrochemical technology has attracted much attention because of its outstanding advantages such as high sensitivity, good practicability, fast response speed, simple operation, low cost and easy miniaturization.
Electrochemical biosensing technology has become one of the most commonly used analysis techniques for environmental pollutants and biomolecules.
For example, Sun et al. developed a sensitive and selective electrochemical dopamine biosensor, which introduced metal phthalocyanine into ZIF-8 by in-situ synthesis method, and realized the determination of dopamine in human urine and fresh pork.
Chen and colleagues developed an electrochemical sensor (Tri-AgNP/L-Cys/MXene) for the detection of serotonin by loading L-cysteine-terminated triangular silver nanosheets onto MXene (two-dimensional transition metal carbides or nitrides).
Qiu and Qu prepared a binary polyaniline-manganese dioxide nanohybrid material for nitrite electrochemical sensing, obtaining a wide linear range and a low detection limit.
glutathione (GSH) is an important biomolecular, a tripeptide composed of glutamine, cysteine and glycine via gamma-amide bonds, which is present in almost every cell of the organism and plays an important role in maintaining normal metabolic processes.
Glutathione helps the immune system function properly.
It also has the functions of anti-oxidation, integration, detoxification, promoting metabolism and maintaining the stability of erythrocyte membrane structure.

Due to the presence of sulfhydryl, glutathione can participate in a variety of important biochemical reactions in the body, protecting enzyme proteins from oxidation and inactivation, ensuring effective energy metabolism and cell utilization.
Glutathione can not only be used as a drug to bind toxins and fight free radical damage, but also as a functional food additive to delay aging and enhance immunity.
Glutathione is also widely present in plants and animals in two forms: reducing (G-SH) and oxidizing (G-S-S-G).
Under physiological conditions, reducing glutathione accounts for the vast majority, and the standard content in human blood is mg/100 g.
The decrease of glutathione content is an early potential signal of apoptosis.
Abnormal glutathione levels are an important risk marker for many diseases, including liver disease, diabetes, Parkinson’s disease, acquired immune deficiency syndrome (AIDS), chronic kidney failure, and cancer.
Accurate and efficient detection of glutathione content in human body is very necessary for the early diagnosis and treatment of various diseases, and also has a guiding role in the development of anti-tumor drugs.
The analytical methods for the determination of glutathione include fluorescence, colorimetry, high performance liquid chromatography, resonance elastic light scattering and capillary electrophoresis.
In particular, electrochemical method has become one of the preferred methods for its unique advantages.
Covalent organic framework (COFs) is a kind of porous crystalline polymer material formed by covalent bonds of light elements such as H, C, N, O and B.
COFs have the characteristics of high specific surface area, natural channel, good stability, diverse structure, simple synthesis method and wide application.
COFs has excellent performance in adsorption separation, photoelectric catalysis, electric energy storage, analytical sensing, medical and other fields.
They have also achieved impressive results in detecting glutathione.
For example, Huang et al. used N-doped Cu-MoF-derived porous carbon (PC) composites to construct CuNPs@NPC modified electrodes for sensitive and rapid detection of glutathione in human cervical cancer (Hela) cell lyases.
MnO2 can quench the fluorescence of triazine COF, while glutathione can restore the fluorescence of COF by reducing the nano-flake MnO2 to Mn2+.
Based on this principle, Qu et al. developed an “open-type” fluorescence sensor for the quantitative detection of glutathione with a LOD of 0.28 μM.
It is of great significance and pioneering to construct an electrochemical sensing platform based on COFs to detect glutathione efficiently and sensitively.
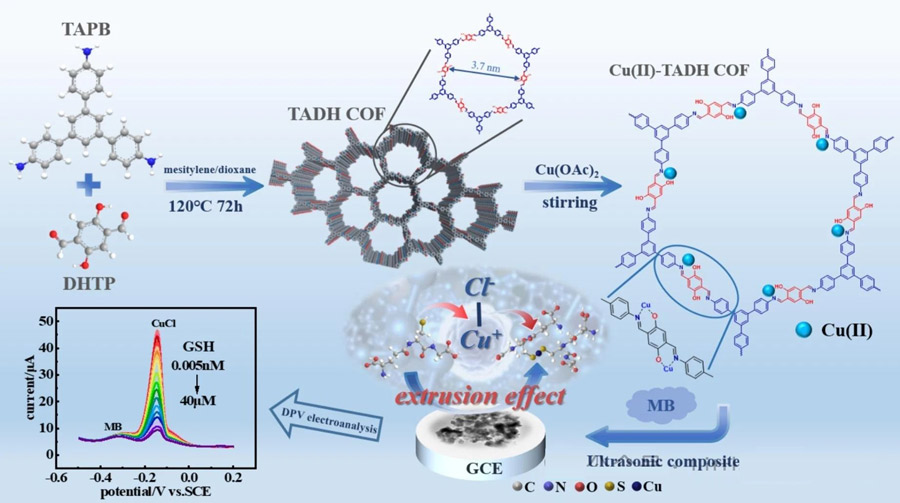
Based on the problems mentioned in the background, Professor Yang Wu from the School of Chemistry and Chemical Engineering of Northwest Normal University, Associate Professor Guo Hao from the School of Chemistry and Chemical Engineering of Northwest Normal University, et al., used a proportional electrochemical biosensor based on Cu (II) modified covalent organic skeleton to detect glutathione specifically.
Ratiometric electrochemical biosensor based on Cu(II) modified covalent organic framework for the ultra-sensitive and specific detection of glutathione was published in Chemical Engineering Journal (IF=13.4).
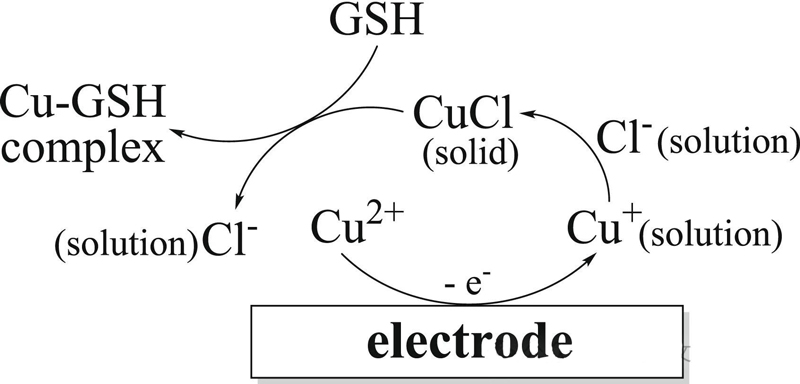
In this study, a Cu2+ modified imine COF was designed and synthesized according to the specific binding of Cu2+ to the sulfhydryl group of glutathione (Cu-S), and it was used to construct the glutathione electrochemical biosensor.
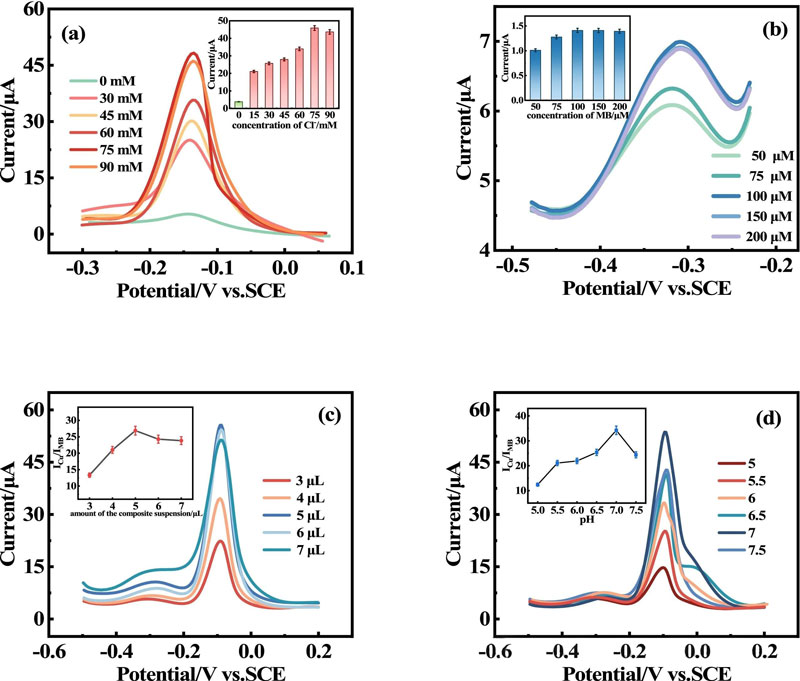
The Cu(II) -Tadh COF modified electrode showed an increased oxidation current in the solution containing Cl-, which was due to the amplification of the solid electrochemical signal of CuCl.
When glutathione is added, CuCl transforms into the non-electrically active species Cu-GSH, immediately triggering a “crowding out effect” that results in a drop in peak current.
The quantitative analysis of glutathione can be realized by using this process.
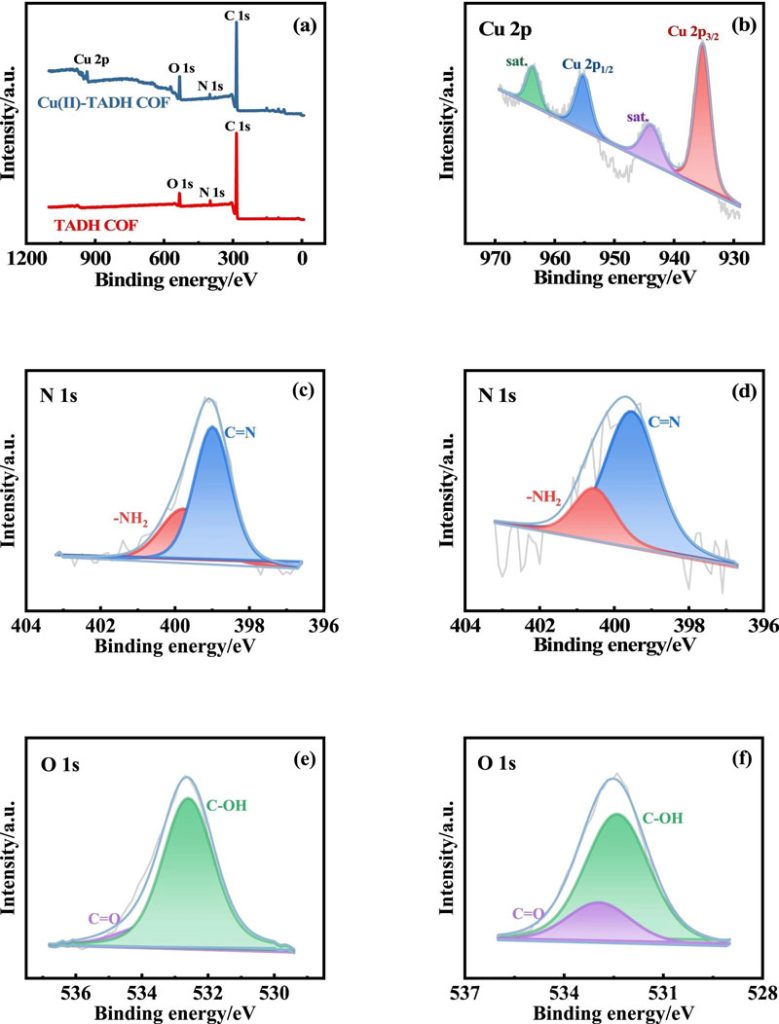
By chelating with N and O sites, Cu(II) is firmly anchored to the pores and surfaces of TADH COF.
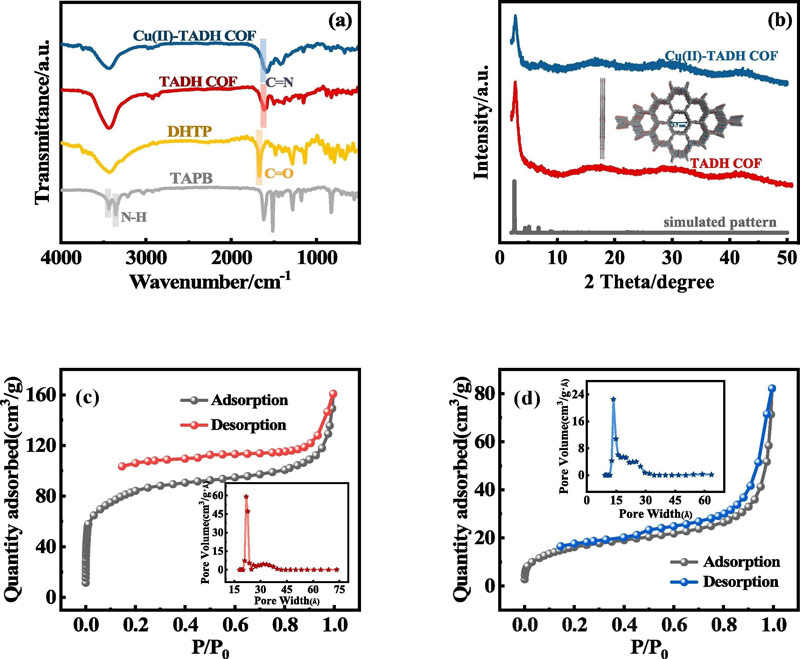
The results of the Brunauer-Emmett-Taylor (BET) experiment, inductively coupled plasma-Optical emission spectroscopy (ICP-OES) and X-ray photoelectron spectroscopy (XPS) clearly show that Cu2+ successfully modified to TADH COF via coordination bonds.
The modification of COF by metal ions significantly improves its electrical conductivity, which is obviously conducive to enhancing its electrochemical performance.
Considering that methylene blue (MB) is a phenthiazine salt that can produce strong and stable electrochemical signals, we used it as an excellent reference material to construct a Cu(II)-TADH COF/MB ratio electrochemical sensor by a simple MB adsorption method.
The self-calibration effect of the sensor effectively improves the accuracy and sensitivity of the detection, and eliminates potential interference.
The electrochemical biosensor also showed excellent stability when detecting human blood, urine and serum samples.
Efficient detection of glutathione is of great medical significance for the early diagnosis of various clinical diseases.
The construction of the electrochemical sensing platform and the detection of glutathione described in Scheme 1.
The study understood to the first attempt at a glutathione electrochemical biosensor based on ratio measurement of COF.
![Figure 4. (a) The scanning rate of bare GCE, TADH COF/GCE, Cu(II) -Tadh COF/GCE and Cu(II) -Tadh COF/MB/GCE in 1.0 mM [Fe(CN)6]3-/4- solution (including 0.1 M KCl) is 50 CV curve and (b) EIS curve of mV s-1 (illustrated as equivalent circuit simulated by Randles); (c) CV response of each modified electrode to 500 nM GSH with a scanning rate of 100 mV s-1 in 0.1 M PBS (including 50 mM Cl-); (d) Cu(II) -TADh COF/MB/GCE CV curve with a scanning rate of 50 mV s-1 in 1.0 mM [Fe(CN)6]3-/4- solution containing 0.1 M KCl. (d) Cu(II) -Tadh COF/MB/GCE CV curves at different scanning rates from 40 mV s-1 to 260 mV s-1 in 1.0 mM [Fe(CN)6]3-/4- solution containing 0.1 M KCl (illustrated as REDOX current vs The linear relationship between v1/2).](https://bio7.net/wp-content/uploads/2025/03/download-4.jpg)
conclusion
Based on the post-synthetic modification of TADH COF by Cu2+, a novel electrochemical sensing platform for the specific detection of glutathion prepared.
A series of characterization results convincingly demonstrate that Cu(II) binds to N and O sites in TADH COF through coordination.
The combination of MB not only improves the conductivity of the electrode material, but also provides a stable signal during the electrochemical sensing process, which can used as an internal parameter to improve the accuracy and sensitivity of glutathione detection.
The large surface area and multiple active sites of TADH COF provide favorable conditions for the loading of Cu2+, while the appropriate pore structure improves the adsorption of MB.
The obtained Cu(II)-TADH COF/MB/GCE has high sensitivity and excellent selectivity for glutathione detection, and has remarkable repeatability and stability.
The designed sensing platform also achieves satisfactory accuracy and recovery rates in the analysis of human blood, serum and urine samples.
This study provides an innovative and convenient strategy for constructing CF-based ratio electrochemical biosensors that can used for ultra-sensitive detection of biological macromolecules and become a new promising solution for clinical diagnosis of relevant diseases, which will accelerate the development of CF-based nanocomposite engineering in the field of biological applications.
Innovation point
(1) Innovation in sensor platform construction
A novel electrochemical sensing platform for the specific detection of GSH prepared by the post-synthetic modification of TADH COF by Cu² +, providing a new strategy for the construction of ratio electrochemical biosensors based on COF.
(2) Performance improvement innovation
The combination of MB not only enhances the conductivity of electrode material, but also improves the accuracy and sensitivity of GSH detection as an internal parameter.
The TADH COF’s large specific surface area, multiple active sites and suitable pore structure enhance the loading capacity of Cu² + and the adsorption capacity of MB, giving the sensing platform high sensitivity, excellent selectivity, significant repeatability and stability for GSH detection.
(3) Application expansion and innovation
The designed sensing platform can achieve satisfactory accuracy and recovery in the analysis of human blood, serum and urine samples, and can used for ultra-sensitive detection of biological macromolecules, which provides a new promising solution for clinical diagnosis of related diseases, and expected to accelerate the development of nanocomposite engineering based on COF in the field of biological applications.
Source: https://doi.org/10.1016/j.cej.2024.152271


