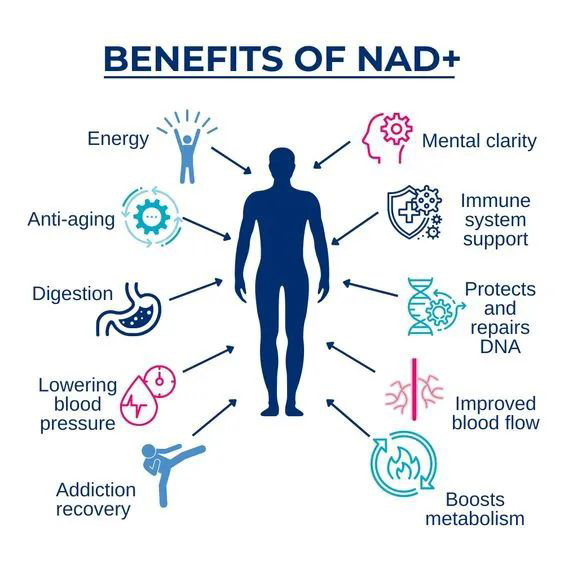
Metro Biotech was co-founded by Professor Sinclair of Harvard University and Professor Apte of the University of Washington. Focuses on the study of NAD+ precursors, especially NMN.
Metro Biotech thinks that if it can target mitochondria, NAD+ precursors could cure more than 50 diseases.
They are now conducting three Phase 2 clinical trials called Super NMN, where the team hopes to explore the greater potential of NMN.
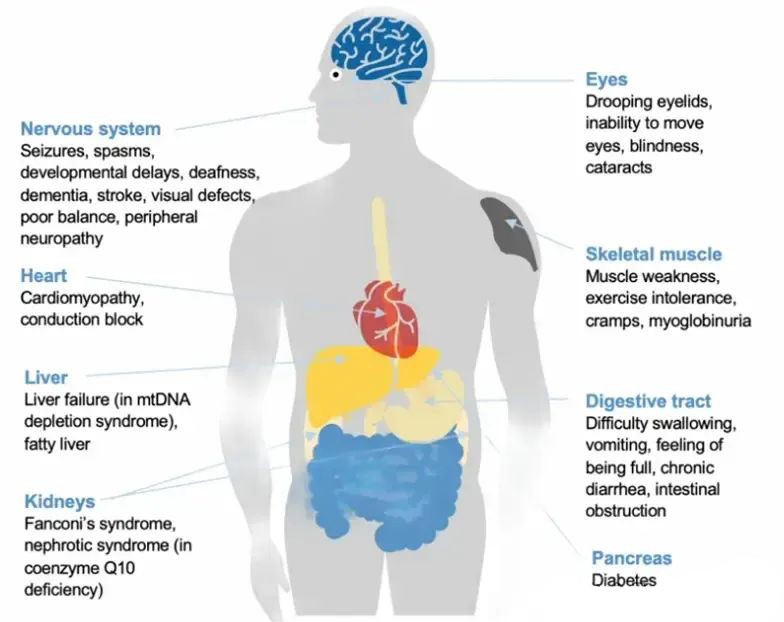
Take, for example, the potential of NAD+ precursors as drugs to treat specific diseases.
The trial is currently in the recruitment phase.
Professor Sinclair said: “After a decade of quiet development, we have developed some new drugs that can regulate NAD+ to treat both rare and common diseases.
For the first time, we are making information from the latest clinical trials public.”
David J. Livingston, CEO of Metro Biotech, said they now have three clinical trials:
1, Look at the effect of NMN on healthy people
The study involved 120 healthy, active volunteers to see if NMN (niacinamide mononucleotide) could improve cardiorespiratory function.
The volunteers underwent detailed physical examinations to ensure their heart and lung function was at normal levels.
During the experiment, the volunteers were randomly divided into two groups, one as an experimental group and the other as a control group. The experimental group consumed a certain amount of NMN daily, while the control group maintained a normal diet.

During the 12-week experiment, the volunteers given regular cardiorespiratory tests, including measurements of heart rate, blood pressure, and lung capacity.
The volunteers will monitored for mental and physical fitness and for any adverse reactions.
At the end of the experiment, the researchers will follow the experimental group of volunteers for a month to see if the effects of NMN on cardiorespiratory function sustained.
2, Look at the effects of NMN on Alzheimer’s patients
The project specifically recruited a number of volunteers suffering from Alzheimer’s disease to see if ingesting NMN (niacinamide mononucleotide) could cross the blood-brain barrier and measure its presence in the cerebrospinal fluid (CSF) to determine whether it could have a positive impact on their condition, thereby helping them improve their current condition.
3, Look at the effect of NMN on patients with diabetic nephropathy (DKD)
This trial included a group of patients with DKD (diabetic kidney disease) to see if ingestion of NMN (niacinamide mononucleotide) could help them to some extent protect against kidney damage and improve related dysfunction.

In the course of the study, the researchers will closely monitor the kidney function indicators of the patients,
as well as the changes in their physical condition before and after taking β-nicotinamide mononucleotide,
in order to obtain conclusive evidence on the impact of NMN on the kidney health of DKD patients.
If these clinical trials are successful, we may see MIB-626 NMN debut in Phase 3 clinical trials.
If all goes well, the drug could approved by the FDA to treat diseases like Alzheimer’s and DKD.