PQQ+ repairs mitochondrial damage, increases the number of mitochondria, promotes body energy production, anti-oxidative stress, anti-aging, antiviral, anti-inflammatory, promotes nerve growth factor synthesis, prevents liver damage, improves lung function, improves immunity, brain cognition, and improves sleep.
Energy factory of PQQ mitochondria
Mitochondria, the engine of life, healthy mitochondria, human cells are healthy.
With the growth of age, air and water pollution, high-sugar, high-fat and high-protein diet, smoking, drinking and staying up late, stress, the emergence of chronic diseases, etc., the human body can have more and more abnormal mitochondria, and many chronic diseases and cancers will come one after another.
PQQ antioxidant
The chemical stability of PQQ enables it to carry out multiple REDOX catalytic cycles.
PQQ is involved in cell signaling in cell proliferation, mitochondria formation and oxidative metabolism.
PQQ’s ability to “REDOX cycle” underlies its unique role in living systems.
PQQ repairs mitochondria
A key role of PQQ is to act directly on the cells that produce energy – the mitochondria. PQQ improves energy generation.
High temperature is the most significant climate feature in summer, but because of the different regions, dry and wet environment, it will produce hot and dry or hot and rainy climate.
PQQ increases mitochondrial count
PQQ not only protects mitochondria from oxidative stress, but also promotes the spontaneous production of new mitochondria within aging cells, a process known as mitochondrial biogenesis or mitogenesis.
The engine of mitochondrial life
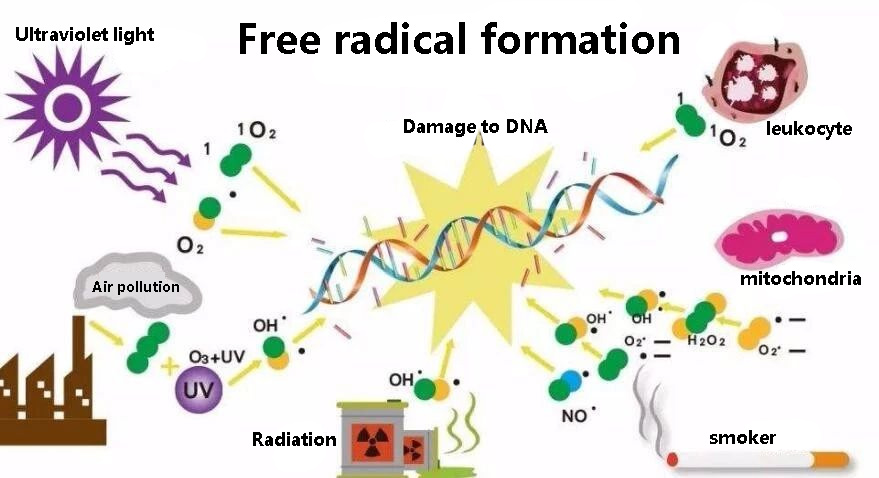
Mitochondria produce a lot of oxygen free radicals and are easily damaged
Mitochondria are not simple. On the one hand, 90% of the oxygen inhaled by the human body is used by mitochondria to produce energy to sustain life.
On the other hand, oxygen is a strong oxidant, it will continue to produce free radicals, too many free radicals, not only damage mitochondria, cell death, but also accelerate human aging, causing various diseases.
Once the mitochondria are sick, the heart, brain, liver, kidneys, pancreas, eyes, sperm, muscles, lungs, bones, and other organs are also sick.
95% of the body’s energy comes from mitochondria
Cells are the basic unit of the human body, the human body has 40 trillion – 60 trillion, the health of the cell directly affects the health of the human body, the cell disease, the human body is sick.
95% of the energy required by human cells comes from mitochondria, which provides life energy for cells. At the same time, mitochondria also regulates the growth and decay of cells, the dynamic balance of calcium ion concentration in cells and gene regulation.
Mitochondria are known as the “engines of life”.
The number of mitochondria determines the metabolic level of the body
Each cell has an average of 300-400 mitochondria, producing 6Kg of ATP per day, more active cells such as brain, muscle, liver cells, kidney cells cardiomyocytes, sperm cells, of which each liver cell has 1000-2000 mitochondria, each kidney cell has 300 mitochondria, each sperm cell has 25 mitochondria.
Especially in the heart, mitochondria account for 30% to 40% of the volume of the heart muscle and provide 70% to 90% of the energy required for cardiac activity.
The number of mitochondria in a cell depends on the metabolic level of the cell, and the more vigorous the metabolic activity of the cell, the more mitochondria.
Many diseases are caused by damaged mitochondria
The more efficient mitochondria you have in your cells, the healthier you will be.
Once there are too many inefficient mitochondria in the cell, or the mitochondrial function is more damaged, it means that the cell is not enough energy support, and various metabolic diseases such as hyperglycemia, hyperlipidemia, gout, obesity, hypertension and so on.
The increase of free radicals and the increase of destruction will damage nerves, bones, organs, blood vessels, etc., accelerate aging, and become one of the inducement factors for a variety of diseases.
Abnormal gene regulation leads to gene mutations that lead to cell mutation or cancer.
1, Fatty liver
Hepatic steatosis and even the development of fatty liver is caused by many causes, mainly due to the disorder of lipid metabolism in liver cells, resulting in the accumulation and clearance of fatty acids and triglycerides in the liver.
Oxidative stress and mitochondrial dysfunction are also considered to be the key mechanisms that accelerate steatosis and lead to fatty liver, and play a key role in the “second blow” of fatty liver.
Mitochondrial dysfunction in turn increases the oxidative stress products such as reactive oxygen species and active nitrogen in the liver, which directly damages mitochondrial DNA, causes the dysfunction of mitochondrial protein synthesis system, leads to the dysregulation of liver cell fat metabolism, and promotes the further development of liver from simple steatosis to steatohepatitis.
2, hypertension
Hypertension is the most important risk factor for cardiovascular diseases, and its main pathological changes include vascular endothelial injury, intima thickening, vascular wall elastic tissue degeneration and vascular smooth muscle cell proliferation.
Studies have shown that mitochondrial dysfunction is closely related to high blood pressure. Angiotensin II can stimulate the production of ROS by mitochondria, oxidate and inactivate the synthesis and release of NO by endothelial cells, resulting in the decrease of endothalium-dependent vasodilation function, the increase of vascular tension and the increase of blood pressure.
Some studies have found that UCP2 gene polymorphism or expression change can cause mitochondrial oxidative phosphoric acid uncoupling, increase arterial blood pressure, and increase plasma renin activity.
3, parkinsonism
Parkinson’s disease (PD) is a common central neurodegenerative disease in middle-aged and older people. The main pathological changes of PD are the degeneration and loss of dopamine (DA) neurons in the dense part of substantia nigra and the formation of lewy bodies in the residual neurons.
A study on the cadavers of Parkinson’s disease patients found that mitochondrial metabolic activity of neurons decreased significantly, oxidative stress was strong, gene mutation, mitochondrial DNA level decreased, ATP production decreased, affecting mitochondrial function and then leading to neuronal cell death, resulting in Parkinson’s disease or deterioration.
4, Myocardial ischemia-reperfusion (Coronary heart disease)
Myocardial ischemia refers to a pathological state in which the blood perfusion of the heart is reduced, leading to a decrease in cardiac support, abnormal myocardial energy metabolism, and unable to support the work of the heart.
Many studies have shown that mitochondrial dysfunction is closely related to ischemia-reperfusion injury.
Excessive oxygen free radicals generated during myocardial ischemia-reperfusion, which on the one hand can damage mitochondria, resulting in mitochondrial ATP synthesis dysfunction, and then lead to ventricular dysdiastole, aggravate acidosis, and promote reperfusion arrhythmia.
On the other hand, oxidative stress can lead to protein and lipid peroxidation, further damaging mitochondria, causing mitochondrial swelling and outer membrane rupture, forming a vicious cycle.
Finally, cardiomyocyte apoptosis or death was induced.
5, tumor
In recent years, with the deepening of research, it has found that the role of mitochondria in the process of cell energy metabolism, oxygen free radical generation and cell apoptosis is closely related to the occurrence of tumor.
Compared with normal tissues, the level of oxygen free radicals in tumor tissues is higher, which will directly lead to mitochondrial dysfunction and further aggravate mitochondrial dysfunction, and finally make tumor cells mainly rely on glycolysis to produce ATP. Aerobic glycolysis is one of the symbols of energy metabolism of tumor cells.
The distribution and quantity of mitochondria in cells also affect the formation of pseudopodia of tumor cells, which in turn affects the migration and invasion ability of tumor cells, and contributes to the sustainable growth of tumor cells.
Studies have shown that mutated mitochondrial DNA has detected in cancer cells such as liver cancer, kidney cancer, and digestive system tumors.
6, Alzheimer’s disease
Alzheimer’s disease (AD) a degenerative disease of the central nervous system mainly characterized by progressive cognitive impairment and memory impairment, and the early manifestation of AD mitochondrial dysfunction.
Damage of mitochondria leads to insufficient energy provided by neurons and damage of neurons, which can also cause dysfunction of synaptic mitochondria, affecting the normal structure and function of synapses, and then lead to cognitive function impairment and memory loss.
Oxidative stress can lead to mitochondrial function damage, and mitochondrial structure and function damage, resulting in synaptic damage, nerve cell apoptosis, and promote the progression of Alzheimer’s disease.
7, Diabetes and complications
Type 1 diabetes: Studies have found that patients with type 1 diabetes mellitus (T1DM) have mitochondrial dysfunction, strong oxidative stress in the body, blocked mitochondrial synthesis and decreased activity, resulting in reduced ATP synthesis of pancreatic β-cells, promoting β-cell apoptosis and resulting in impaired insulin secretion function of β-cells.
Type 2 diabetes: Studies have found that the number and volume of mitochondria in muscle tissue of type 2 diabetes reduced, mitochondrial biosynthesis decreased, and ATP synthesis reduced, which leads to the imbalance of energy supply and demand of the body, and then induces insulin resistance.
Mitochondrial dysfunction can weaken fatty acid β-oxidation, induce a large number of free radicals, resulting in the accumulation of fat metabolites in cells, reduce the sensitivity of fat cells to insulin, inhibit insulin signal transduction and reduce the sensitivity of target tissues to insulin, and ultimately lead to the occurrence of type 2 diabetes.
8, Heart failure
Heart failure a pathological process in which the blood pumping capacity of the heart reduced, resulting in the absolute or relative reduction of the cardiac output that cannot meet the needs of the body. It is the final stage in the development of cardiovascular diseases such as myocardial infarction, hypertension and cardiomyopathy.
In the case of heart failure, the dysfunction of myocardial mitochondria will not only trigger the apoptosis of cardiomyocytes induced by mitochondria, but also increase the production of a large number of free radicals.
The down-regulation of myocardial mitochondrial biosynthesis factors leads to the reduction of mitochondrial DNA content, which not only damages mitochondrial biosynthesis, but also causes mitochondrial oxidative phosphorylation and the reduction of fatty acid oxidation capacity, resulting in insufficient myocardial energy production and aggravating the development of heart failure.
9, Atherosclerosis (AS)
Atherosclerosis (AS) is a non-inflammatory hyperplastic lesion of the intima of the artery. Lipid accumulation in the intima of the artery is yellow in appearance. Lipid metabolism disorders are the basis of atherosclerotic lesions, which often involve large and medium muscular arteries and are the main cause of death from cardiovascular diseases.
Excessive free radicals produced during mitochondrial dysfunction and accumulated mtDNA damage or mutation are closely related to the occurrence and development of atherosclerosis.
Oxidation of AS risk factors such as low-density lipoprotein, high fat and high sugar can inhibit mitochondrial activity, increase the generation of oxygen free radicals, decrease cellular ATP, damage mitochondrial function, promote endothelial damage and atherosclerosis formation, and increase the body’s susceptibility to atherosclerosis risk factors.
What are the causes of impaired mitochondrial function?
Mitochondrial dysfunction mainly refers to energy metabolism disorders caused by mitochondrial membrane destruction, respiratory chain inhibition, enzyme activity reduction, mtDNA damage, etc., which leads to a series of interactive damage processes.
1, Mitochondrial DNA mutation
It can cause mitochondrial dysfunction and can lead to clinical symptoms of diseases related to mitochondrial dysfunction.
2, Mitochondrial permeability is high
ATP synthesis stops; Glutathione depletion, mitochondrial swelling, rupture, release of the inner and outer membrane space causing cell apoptosis or death.
3, Oxygen free radical damage
Oxygen free radicals damage mitochondria and cause mitochondrial dysfunction and apoptosis.
4, Mitochondrial decline
ATP production decreases and the number of mitochondria decreases, causing mitochondrial dysfunction.
GSHWORLD PQQ
GSHWORLD PQQ is a nutritional dietary supplement, its core ingredients contain 20mg of PQQ, PQQ can effectively improve the function of mitochondria, increase the number and quality of mitochondria, promote the efficient production of energy, just like injecting the source of youthful vitality into the cell, so that the cell is full of vitality. Each product also has different ingredients for auxiliary effects, which can be effective in 2-3 weeks.
It is not only suitable for daily health care and anti-aging, but also a gift for visiting relatives and friends to send elders, business and social professionals, and sub-health people!