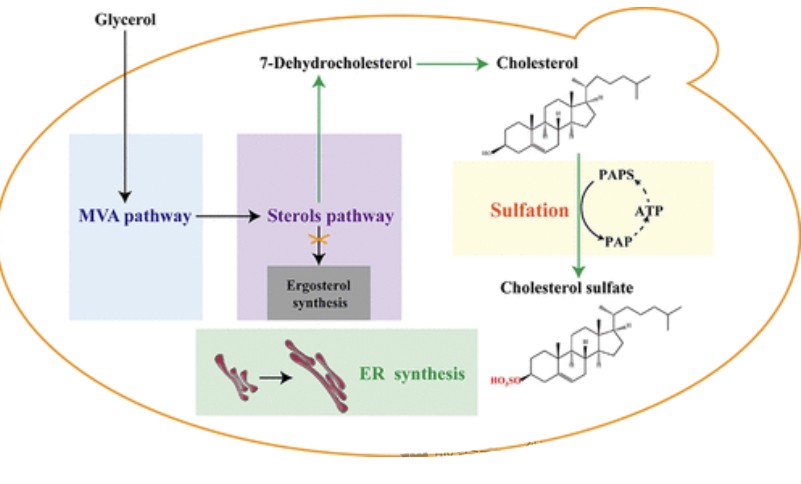
Cholesterol Sulfate (CS), a sulfated derivative of cholesterol, has a wide range of biological and pharmacological activities and has important applications in the medical field.
Since the supply of CS is heavily dependent on the chemical conversion of cholesterol extracted from wool grease, there is an urgent need to develop green cell factories to synthesize CS from simple carbon sources such as glucose and glycerol from scratch.
To improve heterologous steroid production in yeast, common strategies include optimizing three modules, the central carbon metabolism module, mevalonic acid module, and post-squalene module, by disrupting the relevant ERG gene to reduce product consumption in the competitive pathway.
The metabolic flux of squalene was increased by overexpression of essential genes in mevalonic acid module, and the conversion of squalene to sterol was accelerated by enhancement of post-squalene module.
Currently, cholesterol-producing yeast can obtained by introducing the non-genetic sterol C-7 reductase gene (DHCR7 or DWF5) and the sterol C-24 reductase gene DHCR24, combined with the double deletion of the ERG5 and ERG6 genes (Δerg5Δerg6).
Further catalysis of cholesterol to CS by sultyltransferase SULT2B1b has not designed in microbial cell factories.
3 ‘-adenosine-5’ -phosphosulfate (PAPS) is a unique active sulfate group donor in all biological sulfation reactions, and an adequate supply of PAPS is a prerequisite for efficient biosynthesis of these sulfate compounds.
However, enzymatic and microbial synthesis of sulfated natural products remains limited, and there are currently no reports of systematic metabolic engineering to enhance PAPS biosynthesis in vivo to promote microbial production of sulphate compounds.
Therefore, there is an urgent need to explore various metabolic engineering strategies to enhance PAPS supply for the efficient production of sulphate natural products by microorganisms.
Recently, Lian Jiachang et al., Zhejiang University, published a paper entitled “Establishing Komagataella phaffii as a Cell Factory for Efficient” at ACS Sustainable Chemistry & Engineering The Production of Cholesterol Sulfate was the first to report the production of CS by the microbe Phaffia Colum.
The authors achieved 7-DHC biosynthesis in yeast by knocking out competing branch genes and introducing heterologous gene GgDHCR24.
In the same YPD medium, the yield of 7-DHC produced by two inverters of Saccharomyces cerevisiae Syvdb1 was 3 and 19.8 mg/L, respectively, and the yield of 7-DHC produced by one inverter of Saccharomyces farfis DHC01 was 35.6 mg/L.
Cerevisiae fife identified as a more promising model for steroid production.
Next, DHCR7 genes from different sources introduced into DHC01 strain to produce cholesterol, in which DHCR7 strain CH01 from zebrafish introduced to produce 108 mg/L cholesterol, and highly efficient DHCR7 enhanced the metabolic flux of steroid synthesis.
Subsequently, the authors introduced SULT2B1b into the CH01 strain, and the resulting CHS01 strain produced 36.6 mg/L CS and 77.7 mg/L cholesterol.
In order to increase CS production, the authors attempted to enhance the metabolic flux of the CS synthesis pathway by overexpressing tHMG1 (truncated HMGR), ERG20, IDI1 and ACS1, respectively, to obtain strains CHS02, CHS03 and CHS04, with CS production of 87.0, 81.9 and 84.8 mg/L, respectively.
In addition, the authors combined the knockout of endogenous PAPS reductase gene met16 with the overexpression of ATPS and APSK to increase the supply of PAPS and significantly improve CS levels.
The corresponding strains, CHS0501 and CHS0502, produced 148 and 157 mg/LCS.
By overexpressing INO4, the transcription factor encoding gene in phospholipid biosynthesis in CHS0502, the CS titer of CHS0505 was up to 249 mg/L, which was 6.8 times higher than that of the original parent strain.
Finally, the CS and total steroid (7-DHC, cholesterol, and CS) production of strain CHS0505 reached 545 and 621 mg/L, respectively, after 63.5 hours in a batch feeding fermenter, and the total glycerol supply was 450 g.
In summary, in this study, Foal yeast Fife has designed for the first time for the efficient synthesis of CS and is a promising microbial basis for steroid production.