Actl6a-glutathione Axis: The Key Code for gastric cancer cells to escape ferroptosis
Gastric cancer is the fifth most common and the fourth most fatal high-risk cancer in China. Its strong invasiveness and easy metastasis have led to a long-term low survival rate for patients.
To analyze an innovative research from a team at the Sixth Affiliated Hospital of Sun Yat-sen University – it was discovered that a key protein named ACTL6A is actually the “umbrella” that protects gastric cancer cells from death!
Research Background
Gastric cancer (GC) is ranked as the fifth most frequently diagnosed cancer and the fourth most common cause of cancer-related deaths worldwide.
In recent years, the development of high-throughput and genomics technologies has brought the research of GCs into the molecular level.
Molecular expression profiling data have greatly facilitated the identification of candidate gene-driven alterations in GC, such as gene mutations, chromosomal changes, transcriptional alterations, and dysregulated epigenetic modifications.
Actin-like protein 6A (ACTL6A), also known as 53 kDa BRG-1 / human BRM-related factor (BAF53a), is involved in a variety of cellular processes, such as vesicle transport, spindle localization, nuclear migration, cell cycle and chromatin reweight.
ACTL6A has been reported to be associated with the occurrence of various cancers.
Ferroptosis is a regulated form of cell death characterized by the accumulation of iron-dependent lipid hydroperoxides, which differs from apoptosis in morphology and mechanism.
Ferroptosis is associated with a variety of pathological conditions, including acute kidney injury, hepatocyte degeneration and hemoglobin, traumatic brain injury, neurodegeneration and canceration.
Research results
1. ACTL6A is overexpressed in gastric cancer and promotes its progression
The author conducted RNA microarray analysis and genomic enrichment analysis (GSEA) and found that the mRNA level of ACTL6A in cancer tissues was higher than that in normal tissues.
And the expression level of ACTL6A mRNA was verified in 21 pairs of tumor tissues and normal tissues.
To further explore the role of ACTL6A in gastric cancer, the author constructed a cell line with this gene knocked out and found that constructing ACTL6A inhibited the proliferation of gastric cancer cells, while overexpression did the opposite.
The scratch and transwell assays demonstrated the ability of ACTL6A knockout to inhibit the lateral and longitudinal migration of gastric cancer cells.
Knockdown of the ACTL6A group inhibited the growth of patient-derived organoids, and the organoids of ACTL6A KD were compact in morphology and had no lumen. Knockdown of ACTL6A significantly reduced the growth of xenografts.
ACTL6A is highly expressed in GC and promotes the progression of gastric cancer.
2. ACTL6A reprograms glutathione metabolism to maintain malignant progression of GC
To determine the role of ACTL6A in GC, gene set enrichment analysis (GSEA) conducted, in which glutathione metabolism significantly enriched.
The same results obtained from the other two datasets.
Glutathione is an antioxidant that protects cancer cells from oxidative stress through the Reduced Glutathione/GSSG cycle, thereby reducing intracellular ROS levels and converting NADPH to NADP+, which promotes tumor growth.
After the knockout of ACTL6A, the ratios of Reduced Glutathione/Oxidized Glutathione and NADP+ /NADPH both decreased.
Knockout of the ROS level in this genome significantly increased.
NAC treatment restored the inhibition of proliferation and reduction of organoids caused by knockdown of ACTL6A, and restored the cystic structure of the organoids.
To determine the effect of ACTL6A on the in vivo function of tumors, the authors constructed a GC xenograft mouse model experiment.
It found that knockout of ACTL6A significantly reduced tumor growth and could reversed by NAC.
The above results indicate that ACTL6A accelerates glutathione synthesis and reduces ROS levels to support GC tumor growth.
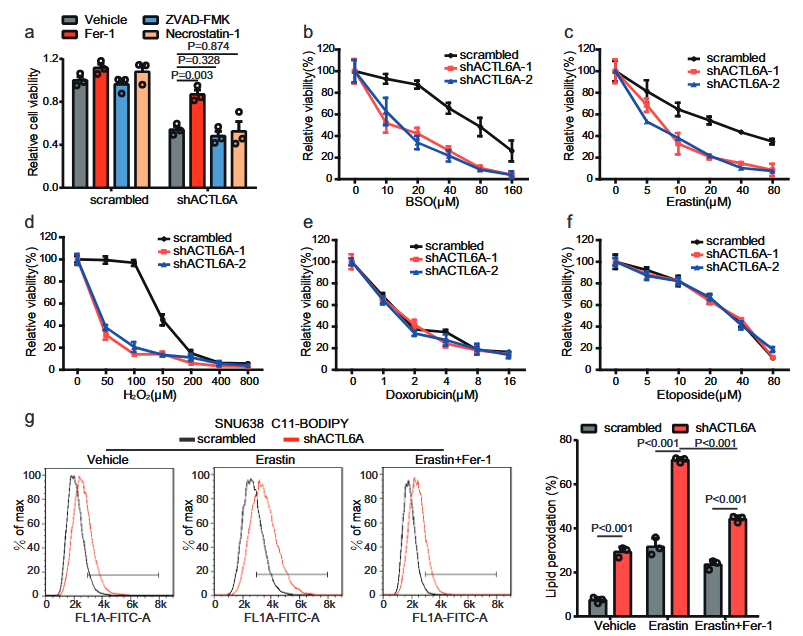
3. ACTL6A inhibits ferroptosis in gastric cancer cells
As ferroptosis is related to glutathione activity, the authors speculated whether ACTL6A inhibits ferroptosis in GC cells.
By applying multiple inhibitors, it found that only ferroptosis inhibitors could reverse the inhibitory effect of ACTL6A knockout on the proliferation of GC cells.
Moreover, knocking out ACTL6A made GC cells sensitive to ferroptosis inducers but insensitive to other compounds.
BODIPY-C11 staining revealed that after knockout of ACTL6A, the level of lipid peroxidation increased, and Fer-1 treatment restored the PDO growth inhibition induced by ACTL6A knockout and restored the cystic structure of organoids.
All of the above could reversed by fer1.
Through IHC staining of xenograft tumor tissues, it found that knockout of ACTL6A significantly reduced the level of Ki67 and increased the level of lipid peroxidation markers.
The above results indicate that knockout of ACTL6A promotes ferroptosis in GC cells.
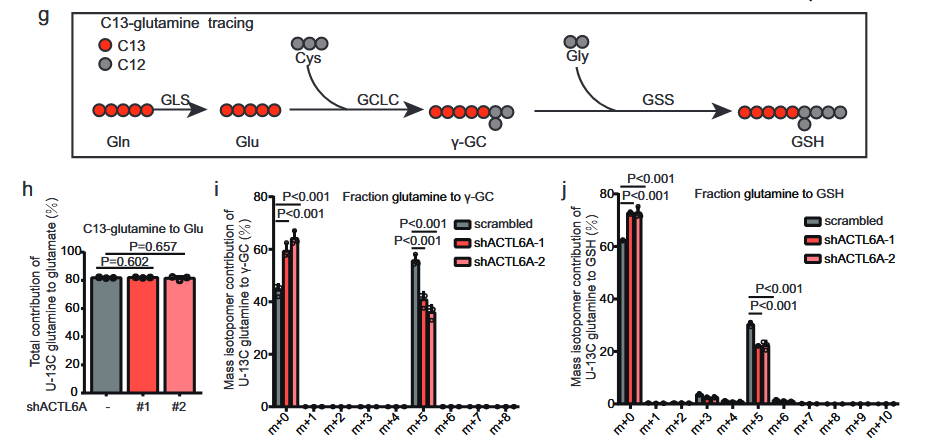
4. ACTL6A mainly affects synthesis of glutathione by up-regulating synthesis of γ -glutamyl cysteine
Subsequently, the author investigated the metabolic processes of U-13C glucose and U-13C glutamine by liquid chromatography-mass spectrometry (LC-MS).
The results showed that the total contribution of U-13C glucose to glutamic acid, serine or glycine did not change significantly.
The levels of M+ 2-labeled [13C]-γ-GC and M+2 and M+ 4-labeled [13C]-GSH decreased in cells expressing shACTL6A, while in cells expressing shACTL6A, M+ 5-labeled [13C]-γ-GC and M+ 5-labeled [13C]-GSH decreased.
This confirms that ACTL6A mainly affects the synthesis of GSH by regulating the synthesis of γ-GC.
The above results indicate that ACTL6A mainly affects the synthesis of glutathione by regulating the synthesis of γ -glutamyl cysteine.
5. ACTL6A regulates GCLC at transcriptional level through NRF2
Knockdown of ACTL6A inhibits the expression of GCLC in xenograft tissues.
Then, the author overexpressed GCLC on the basis of knockdown ACTL6A and found that GCLC reversed the inhibition of cell proliferation.
Through ROS detection, the ROS levels in cells with overexpressing GCLC by knocking down ACTL6A altered.
The effect remained significant when treated with ferroptosis inducers and reversed by NAS-ROS scavengers.
C11-BODIPY staining indicated that overexpression of GCLC restored the knockdown of ACTL6A lipid peroxidation levels.
The phenotype remained significant when treated with erastin and reversed by Fer-1.
To explore how ACTL6A regulates GCLC, the authors conducted chromatin immunoprecipitation high-throughput sequencing (ChIP-seq) on endogenous ACTL6A in GC cells (Figure A shows a binding peak TSS upstream of the GCLC transcription start site).
Using IgG as a control, a sequence highly homologous to the NrF2-binding motif observed through the website JASPAR.
The authors hypothesized that ACTL6A might synergize with NRF2 to regulate GCLC expression.
The results showed that when NRF2 knocked out, the inhibitory effect of ACTL6A KD on GCLC expression weakened.
However, when ACTL6A is knocked out, NRF2 KD does not inhibit GCLC expression.
Subsequently, qRT-PCR performed, and it found that both ACTL6A and NRF2 interacted with the predicted binding sites near GCLC TSS.
Knocking down NRF2 weakened the interaction between ACTL6A and the GCLC promoter, and knocking down ACTL6A also weakened the interaction between NRF2 and the GCLC promoter.
The interaction between NRF2 and ACTL6A in GC cells confirmed by the co-IP experiment.
NRF2 is a transcription factor for GCLC. ACTL6A works in conjunction with NRF2 to influence the binding of NRF2 to the GCLC promoter, thereby regulating the expression of GCLC at the transcriptional level.
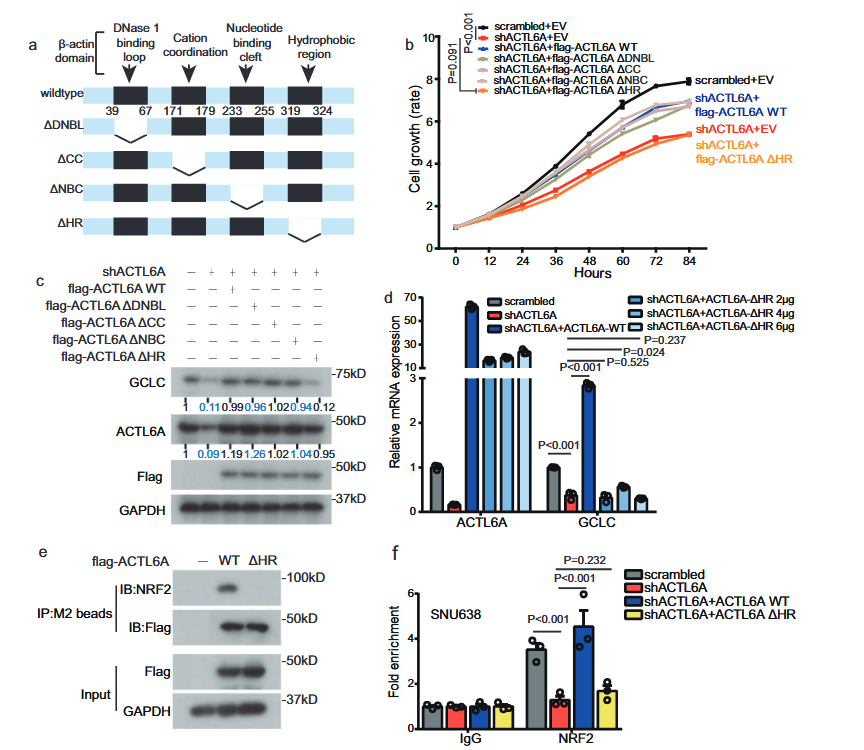
6. HR domain of ACTL6A is crucial in regulating GCLC and ferroapoptosis
Subsequently, in order to identify which part of ACTL6A has the greatest impact on regulating GCLC and ferroptosis, four deletion mutants, ΔDNBL, ΔCC, ΔNBC and ΔHR, fabricated by deleting different sites bound to β -actin and transfected into knockdown ACTL6A cells.
We found that wild-type (WT) ACTL6A and ΔDNBL, ΔCC and ΔNBC ACTL6A mutants reversed the inhibition of cell proliferation and the reduced expression of GCLC protein induced by knockdown of ACTL6A, but the ACTL6A HR deletion mutant did not exhibit this recovery effect.
It is inferred that the HR domain of ACTL6A plays a crucial role in the regulation of GCLC.
co-IP performed and it found that the ACTL6AΔHR mutant did not bind to NRF2.
Chromatin immunoprecipitation assay indicated that, compared with WT ACTL6A, the ACTL6A HR deletion mutant had no restorative effect on the binding of NRF2 to the GCLC promoter.
The author infers that the HR domain of ACTL6A has an impact on ferroapoptosis.
ROS detection and C11-BODIPY staining performed in advance, and it found that the WT wild-type ACTL6A reversed the increase in ROS levels and lipid peroxidation levels, while the ACTL6A HR deletion mutant did not reverse these high levels.
7. clinical relevance of ACTL6A-GCLC-glutathione metabolic axis in ferroptosis of gastric cancer cells
The author implanted fresh primary tumor samples resected from gastric cancer patients into mice and injected PBS or the GCLC inhibitor BSO into the mice.
It found that administration of BSO in the group with high expression of ACTL6A could alleviate tumor progression. Low expression is the opposite.
High GCLC expression is also present in ACTL6A-high PDX tumors.
BSO reduced the intensity of Ki67 staining in tumors with high ACTL6A expression and increased the intensity of 4-HNE staining, but this trend not observed in PDX tumors with low ACTL6A expression.
Through ROS and C11-BODIPY staining of frozen sections of PDX tumors, it found that the ROS levels and lipid peroxidation levels of PDX tumors with high ACTL6A expression were lower, and significantly increased after BSO treatment.
The above results indicate that compared with tumors with low expression of ACTL6A, those with high expression of ACTL6A are more dependent on glutathione metabolism and more sensitive to ferroptosis inducers or GCLC inhibitors.
Analysis of paired samples from GC tissues and adjacent normal mucosa revealed that the GC samples exhibited high expression levels of ACTL6A and GCLC proteins.
The clinical relevance of these findings further evaluated by analyzing the expression of ACTL6A and GCLC using a tissue microarray containing 184 GC tissue samples.
Kaplan-Meier analysis indicated that high levels of ACTL6A and GCLC associated with poor overall survival.
Moreover, the expression of GCLC significantly positively correlated with ACTL6A in GC, as shown in the analysis of 184 GC samples in the microarray.
Summary
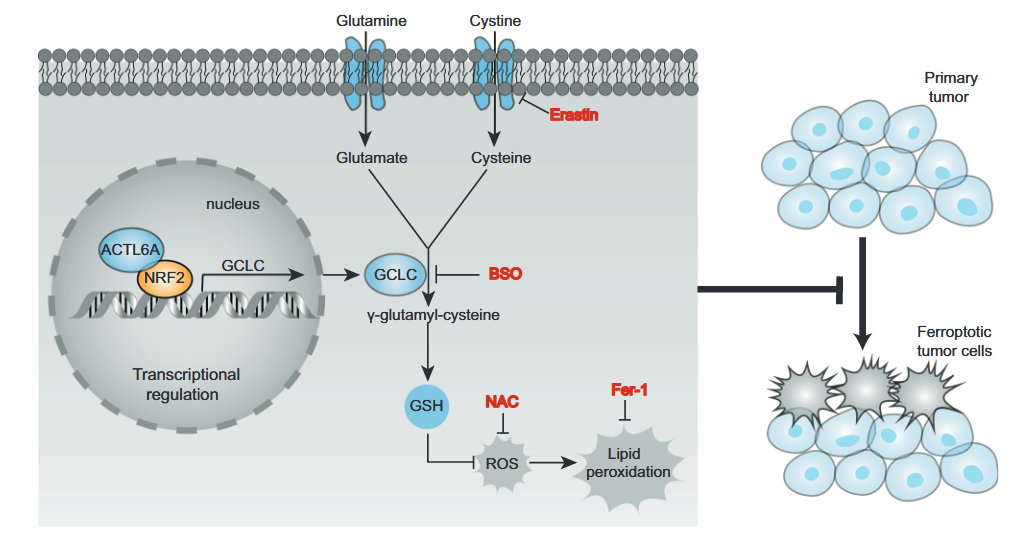
ACTL6A (actin-like protein 6A) plays a key carcinogenic role in gastric cancer. This protein, as a transcriptional co-regulatory factor, promotes the synthesis of glutathione (GSH) by synergistic action with the antioxidant transcription factor NRF2, upregulating the expression of the rate-limiting enzyme GCLC for glutathione synthesis.
This mechanism significantly reduces intracellular reactive oxygen species (ROS) levels and inhibits ferroptosis, a programmed cell death driven by lipid peroxidation.
Studies have confirmed that inhibiting ACTL6A or GCLC can induce ferroptosis in gastric cancer cells and significantly inhibit tumor growth.
Clinical analysis further revealed that the high expression of ACTL6A and GCLC associated with a poor prognosis in patients with gastric cancer, indicating that they could serve as potential therapeutic targets.
This study revealed the core role of the ACTL6A-GCLC-GSH metabolic axis in maintaining the malignant progression of gastric cancer, providing a theoretical basis for the therapeutic strategy targeting ferroptosis.
Advantages
For the first time, the molecular cascade revealed: The complete pathway by which ACTL6A (chromatin remodeling protein) inhibits ferroptosis by recruiting NRF2 to co-activate GCLC gene transcription and promoting glutathione (GSH) synthesis clarified.
It is clear that BSO (glutathione inhibitor) has a significant therapeutic effect on tumors with high expression of ACTL6A (PDX tumor volume reduction >60%), but is ineffective against low expression, achieving therapeutic stratification.
Disadvantage
Although targeting the ACTL6A-HR domain proposed, no inhibitors tested, and BSO has high clinical toxicity (liver damage), so safer drugs need to developed.

