Metabolic processes in cells are constantly influenced by various environmental factors. glutathione
Many small metabolites (such as amino acids, certain coenzymes, and metal ions) need to be maintained at appropriate physiological concentrations to ensure normal cellular metabolic function.
As a result, mammalian cells have evolved many molecular mechanisms for sensing and regulating metabolites. However, previous studies of metabolic sensing mechanisms have focused on metabolites in the cytoplasm, and mammalian cells have many membrane-wrapped organelles.
These compartmentalized subcellular structures create biochemical environments distinct from the cytoplasm. How metabolites in these subcellular structures are perceived and regulated by cells remains poorly understood.
Glutathione is an important antioxidant in cells and plays an important role in cellular iron metabolism and detoxification of allogenic substances.
In mitochondria, endoplasmic reticulum and other subcellular structures, glutathione is involved in important physiological processes such as iron-sulfur cluster synthesis and protein folding, but its synthetic pathways are all located in the cytoplasm.
The transport and regulatory mechanisms of glutathione in these organelles are not well understood.

On November 2, 2023, Kivanc Birsoy research group of Rockefeller University in the United States published a research article entitled Autoregulatory Control of Mitochondrial Glutathione Homeostasis in Science. The feedback regulation mechanism of glutathione balance in mitochondria revealed.
Previous studies have shown that reduced glutathione enters the mitochondrial matrix via a transporter SLC25A39 on the inner mitochondrial membrane (Wang et. al Nature 2021 BioArt 2021-10-28 Nature|SLC25A39).
The authors note that both drug inhibition of glutathione synthesis or knockout of GCLC, a key enzyme in the glutathione synthesis pathway, caused a strong increase in SLC25A39 protein, raising the level of this transporter by some feedback mechanism in the cell.
SLC25A39 protein expressed by exogenous promoter still retains the GSH response, suggesting that SLC25A39 accumulation is independent of transcriptional regulation.
The authors found that SLC25A39 protein is very unstable under physiological conditions (half-life is only about 15 minutes); Reducing the content of intracellular glutathione significantly improves the stability of SLC25A39 protein and leads to its accumulation in mitochondria.
Because SLC25A39 protein connects two intracellular partitions of the mitochondrial matrix and the cytoplasmic/intermembrane space, we designed mitochondria-targeting glutathione-specific gamma-glutamyl cyclotransferase 1 (MitoCHAC1) to specifically manipulate glutathione levels in the mitochondrial matrix.
The authors observed that reducing glutathione in the mitochondrial matrix was sufficient to induce the accumulation of SLC25A39. In addition, GSH still promotes the degradation of SLC25A39 protein in mitochondria where cytoplasmic components have removed by immunopurification.
These data suggest that a mechanism for sensing intracellular GSH exists in the mitochondrial matrix and controls the stability of the transporter SLC25A39.
The authors hypothesize that some mitochondria-specific protein degradation system controls SLC25A39 levels.
To this end, we designed a sgRNA library for all known mitochondrial proteases and a CRISPR screening system based on immunofluorescence staining and flow cytometry.
The results showed that the stroma-facing AAA-atpase protease AFG3L2 was responsible for the rapid degradation of SLC25A39.
Co-immunoprecipitation experiments have shown that AFG3L2 and SLC25A39 interaction is highly sensitive to glutathione: inhibition of glutathione synthesis blocks AFG3L2 binding to SLC25A39, thereby preventing the rapid degradation of SLC25A39 and leading to its accumulation, and feedback increases the rate of mitochondrial take-up of glutathione.
Analysis of the AlphaFold predicted structural model of SLC25A39 showed that a ring region on the mitochondrial matrix side of SLC25A39 mediated its interaction with AFG3L2 and its response to glutathione.
Further analysis showed that four cysteine residues in this segment are critical for sensing glutathione in the mitochondrial matrix: the introduction of mutations in these residues is sufficient to completely prevent the accumulation of SLC25A39.
Cysteine residues play an important role in sensing REDOX balance and binding cofactors.
To reveal how these cysteine resists sense glutathione, the authors extended a previous CRISPR screening experiment: The authors designed a CRISPR sgRNA library that included all known mitochondrial localization proteins and screened for genes affecting SLC25A39 accumulation in cells with impaired glutathione synthesis.
The results showed that the accumulation of SLC25A39 strongly influenced by several genes in the synthesis pathway of disulfide clusters. The accumulation of SLC25A39 significantly inhibited after acute knockout of ISCU, HSCB or NFS1.
Further analysis showed that the diiron disulfide cluster synthesis prevented SLC25A39 from recognized by AFG3L2 when GSH synthesis blocked, thus helping it to remain stable.
Ferrodisulfide clusters usually bound by four cysteines; The authors hypothesized that the four cysteine residues mediating GSH in SLC25A39 may directly bind to the ferrodisulfide cluster when glutathione reduced.
Therefore, we synthesized polypeptide fragments containing the sequences near the above cysteine. Uv-vis absorption spectrum and Mossbauer spectrum analysis showed that this peptide fragment can indeed bind to a diferrodisulfide cluster under in vitro conditions.
In cells, the radioactive 55Fe tracer showed that a decrease in GSH led to a strong increase in SLC25A39 binding iron signaling; And this phenomenon is dependent on key cysteine residues on the mitochondrial stromal side of SLC25A39.
These results suggest that SLC25A39 senses GSH in the mitochondrial matrix by binding to a glutathione-sensitive diiron disulfide cluster.
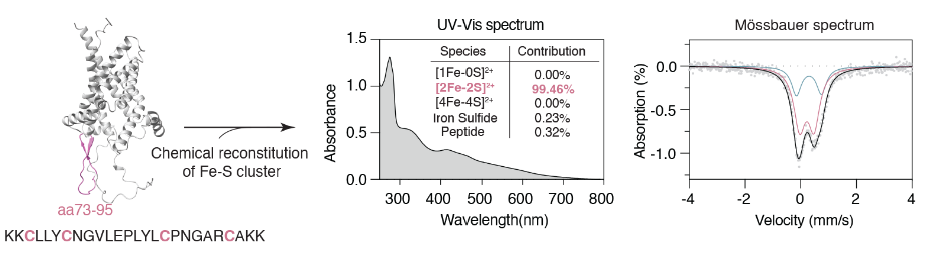
The authors observed that glutathione can affect the binding of cysteine residues to the diiron disulfide cluster in the aforementioned polypeptide fragment in vitro, possibly leading to exposure of this segment to recognition and degradation by AFG3L2.
In addition to antioxidant effects, glutathione considered to an important ligand for iron ions within cells.
The synthesis of iron and sulfur clusters is crucial for regulating the balance of iron metabolism in cells.
Therefore, the authors hypothesized that the glutathione receptor mechanism involved in SLC25A39 also plays a role in iron metabolism in mitochondria.
Increased mitochondrial iron uptake through overexpression of mitochondrial iron transporters SLC25A37 or SLC25A28 also leads to accumulation of SLC25A39 and a compensatory increase in mitochondrial glutathione. Conversely, iron chelation can reduce the accumulation of SLC25A39.
The levels of iron and glutathione in mitochondria disturbed by various means, and the results all showed that the accumulation of SLC25A39 controlled by the relative balance of iron and glutathione.
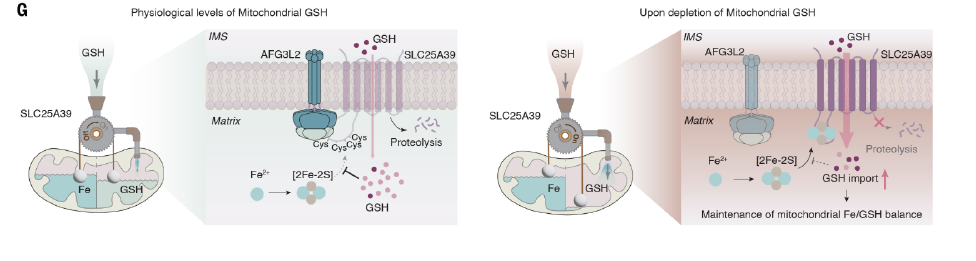
This mechanism appears to help protect mitochondrial function from iron overload; Proteomic analysis showed that when SLC25A39 is absent, mitochondrial iron overload leads to more severe disturbances in the assembly of protein complexes such as respiratory chain and mitochondrial translation.
Taken together, this work reveals a feedback regulation mechanism of glutathione balance in mitochondria. Glutathione supplier
Mitochondrial glutathione and iron metabolism have significant changes in oxidative stress response, erythropoiesis and other physiological processes. The role of this feedback regulation mechanism in the above process not fully understood.
In addition, many other metabolites selectively enriched in each organelle, and whether there are corresponding metabolic sensing mechanisms in these subcellular structures needs to further explored.
Original source:
Liu Y, Liu S, Tomar A, Yen FS, Unlu G, Ropek N, Weber RA, Wang Y, Khan A, Gad M, Peng J, Terzi E, Alwaseem H, Pagano AE, Heissel S, Molina H, Allwein B, Kenny TC, Possemato RL, Zhao L, Hite RK, Vinogradova EV, Mansy SS, Birsoy K. Autoregulatory control of mitochondrial glutathione homeostasis. Science. 2023 Nov 2:eadf4154. doi: 10.1126/science.adf4154. Epub ahead of print. PMID: 37917749.

