Chinese scholars led the team to manipulate the biofilm structure with DNA origami to achieve the precise delivery of macromolecular drugs
In the field of biomedicine, how to achieve accurate and efficient delivery of drugs has always been a key problem for scientists to explore.
In recent years, with the rapid development of DNA nanotechnology, a new solution to this problem has been brought.
Recently, Laura Na Liu, director of the Second Institute of Physics at the University of Stuttgart and a researcher at the Max Planck Institute for Solid State Research, successfully manipulated the structure and function of biofilms using DNA origami structures, making it easier to deliver large doses of therapeutic drugs into cells.
The research is published online in Nature Materials, Entitled “Morphology remodelling and membrane channel formation in synthetic cells via reconfigurable DNA nanorafts”.
One of Liu’s main research areas is DNA nanotechnology.
She specializes in DNA origami structures, which are strands of DNA folded using shorter, custom-made DNA sequences.
The team employed a DNA origami structure as a reconfigurable nanorobot that can reversibly change its shape and affect its surroundings on the micron scale.
In this study, the researchers constructed a synthetic cell model consisting of signal-responsive DNA nanocrafts, biological pores, and giant monolayer vesicles (GUVs).
Among them, DNA nanofraft, as a DNA structure that can reshaped on the nanoscale, is based on DNA origami design.
A GUV is a simple, cell-sized structure that mimics a living cell.
Through the interaction, the DNA nanofraft exerts reversible deformation and restoration on GUV morphology, translating the conformational changes of the DNA raft at the nanoscale into the engineering behavior of the GUV at the microscopic scale to reshape its membrane shape, perforate lipid membranes, and regulate cargo flux.
Specifically, when the DNA nanofraft hatched with the GUV and reached equilibrium, the DNA nanofraft changed from a nearly square structure to a slender rectangular structure under the action of “unlocking the DNA strand,” a process accompanied by a significant change in aspect ratio.
This causes the GUV film to deform, changing it from spherical to concave and uneven.
Subsequently, the addition of “locked DNA strands” restored the DNA nanofraft to its initial, nearly square state.
In this process, the GUV changes back into a spherical shape.
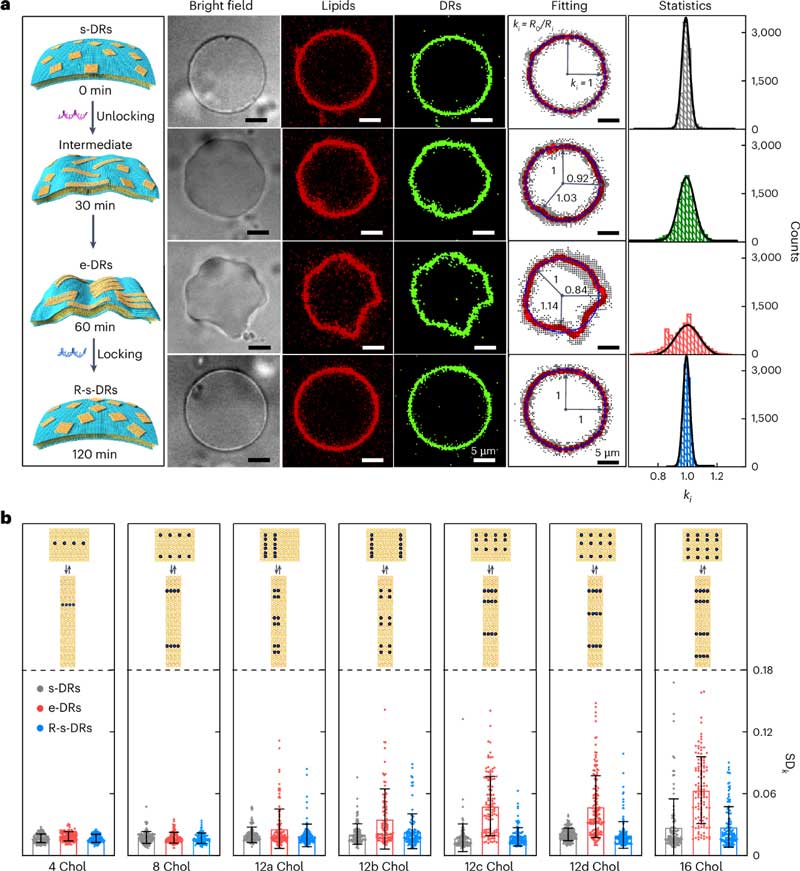
To build a cell model composed of mixed modules, the researchers selected the bacterial outer membrane protein OmpF as a biopore, with a pore size that allows molecules <600 Da to pass through.
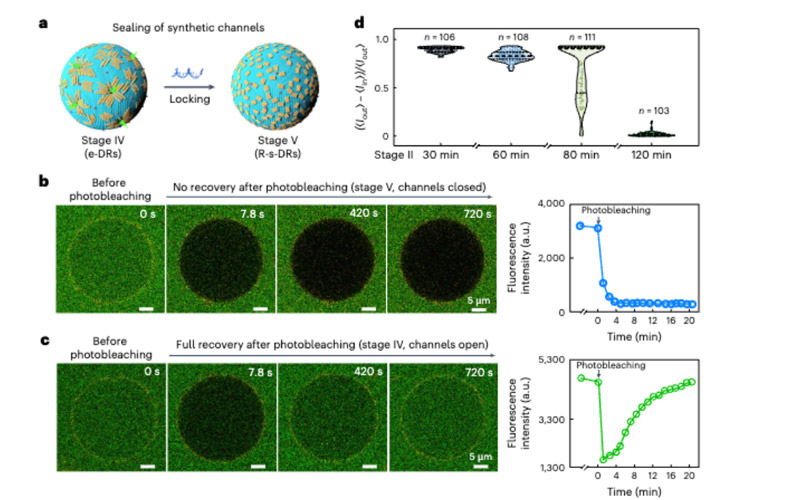
Importantly, these channels can also programmatically sealed and gated as needed.
In closed enzyme cascade experiments, large molecular weight reactants usually pre-packaged directly during GUV production or injected into vesicles.
The researchers took advantage of the different sizes and functions of the synthetic channels and OmpF, where different reactants progressively transported into the GUV, thereby achieving a high degree of spatiotemporal control of the enzyme cascade within the GUV.
It is worth noting that these self-assembled DNA nanocrafts can synergistically interact with biogenic pores to penetrate cell membranes and form artificial channels during GUV morphological recovery.
These channels are large enough to allow large molecular weight substances, such as proteins up to about 70kDa, to pass through.
Moreover, these synthetic channels are controllable and can sealed on demand by changing the conformation of the DNA nanraft.
This property allows researchers to precisely control the movement of substances into and out of cells, providing unprecedented precision for drug delivery.
In living cells, the flow of macromolecules across membranes mainly regulated by protein pores, which are often difficult to design and have a narrow size range of up to 5 nanometers.
Going beyond this size limit and overcoming some of the limitations of protein pores has profound biological and technical implications.
This study does not require complex and synergistic biological processes through protein pores or through substrate-specific protein translocation enzymes.
Instead, the synthetic channels formed can facilitate the direct transport of large bioactive substances.
In addition, given the modularity of DNA origami, DNA rafts can functionalized with different parts to accurately identify and programmatically puncture diseased cells, thereby activating apoptosis or controlling the release of synthetic cell-coated therapeutic agents or cytotoxic drugs.
The researchers envision that future work along these lines could inspire new solutions to unmet bottlenecks in cell delivery and release of large biological entities such as protein complexes, small synthetic vesicles, therapeutics and personalized medicine or cytotoxic drugs.
All in all, this research opens up new frontiers for targeted drug delivery.
As an innovative tool, DNA nanorobots provide a new perspective for us to deeply understand cell behavior and develop novel therapeutic strategies, and its believed that in the near future, DNA nanorobots will play an important role in clinical treatment and bring new well-being to human health.
References:
- https://phys.org/news/2025-01-dna-nanorobots-artificial-cells-tool.html
- https://www.nature.com/articles/s41563-024-02075-9