There have been many reports on glutathione biosynthesis. It mainly focuses on the synthesis pathway catalyzed by the two-step enzymes gamma-glutamylcysteine Synthetase (gamma-GCS) GSHI and Glutathione Synthetase (GS) GSHII. However, the evolutionary process of GSHI and GSHII is very complex, and there are many synthetic pathways for glutathione biosynthesis in vivo.
Overview of glutathione production methods
There are four main ways to produce GSH: direct extraction, chemical synthesis, enzyme conversion and microbial fermentation.
The most important method to produce GSH is microbial fermentation.
Microbial fermentation method is to produce GSH by using the reaction system of microorganisms themselves with cheap microbial raw materials as substrate.
Microbial fermentation not only has low cost, but also avoids environmental pollution, so it is a popular method to produce GSH.
There are also attempts to use synthetic biology technology to establish in vitro ATP cycle technology coupled enzymes to promote the production of GSH, the application of this technology may replace the traditional microbial fermentation production process, is expected to promote the wide application of GSH.
The most commonly used microbial strains are yeast and Escherichia coli.
In general, in order to increase the GSH yield of microbial fermentation,
genetic modification can be used to modify strains and optimize the fermentation conditions of strains.
Random mutation is one of the earliest techniques of gene modification.
With the development of genetic engineering, metabolic engineering, synthetic biology and other disciplines,
the traditional mutagenesis breeding has been gradually replaced by the synthesis of target products by means of molecular biology.
In prokaryotes, genes encoded by the glutathione synthase genes (GSH1 and GSH2) can synthesize two enzymes of GSH: γ⁃GCS and GS,
and the expression levels of these two genes in Escherichia coli affect the production of GSH.
Due to the feedback inhibition of GSH on gamma ⁃GCS, excessive intracellular GSH accumulation is limited.
Since the synthesis of GSH is an energy-intensive reaction, Xu et al. constructed ATP6 mutants of Candida candida by genome integration in order to improve the synthase activity.
The activity of ATP synthase was increased, and the GSH content of the mutant was increased by 28.7%.
On the basis of mutant strain GS115⁃M7 from Pichia Pastoris, Jiang Qiuqi integrated the recombinant plasmid carrying GSH1 and GSH2 genes into the genome of the target strain,
and finally obtained a stable genetic high-yield strain GS115⁃M710,
the yield increased by 45.5% compared with the pre-recombinant strain.
Gene modification can improve the ability of single bacteria to synthesize GSH,
but the number of cells per unit volume needs to be further increased.
The optimization of GSH fermentation mainly focuses on the culture conditions, the addition amount of three precursor amino acids and the cofactor ATP.
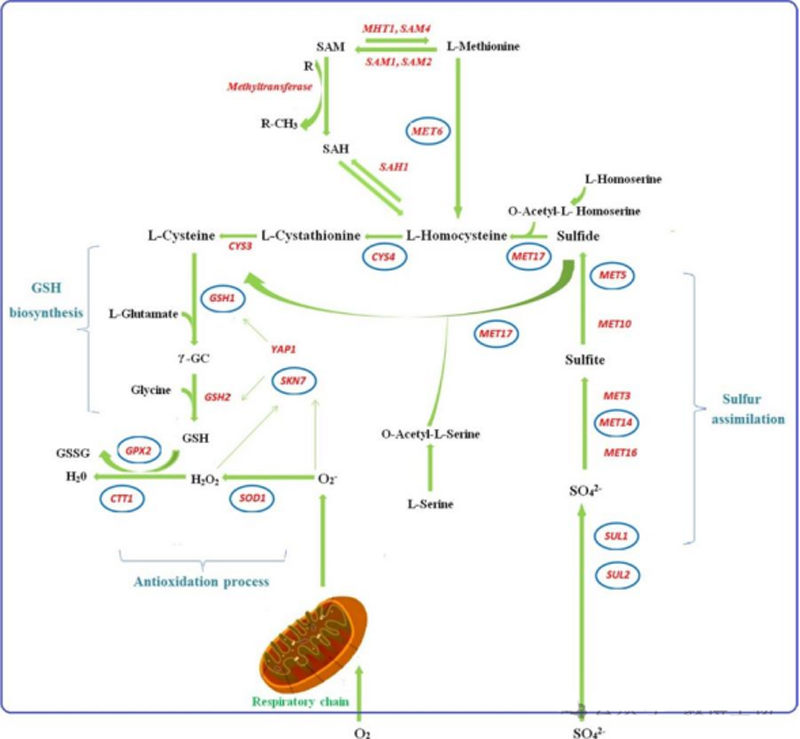
Metabolic pathway of glutathione in Saccharomyces cerevisiae
Saccharomyces cerevisiae is the most important strain for glutathione production due to its rapid growth in low-cost media and its potential to accumulate high levels of GSH in cells.
GSH is an intracellular product of yeast, and its production can be increased in two ways:
by increasing the cell biomass (the so-called high cell density culture technique) and by increasing the GSH content in yeast, which can be achieved by regulating amino acids.
It is easier to increase biomass by fermentation than to increase intracellular GSH content. In addition, the increase of intracellular GSH level is also conducive to downstream extraction processing.
The synthesis and catabolism of GSH in Saccharomyces cerevisiae cells have been studied, which is mainly metabolized by the γ-glutamyl cycle in the cell.
Breeding of high-yielding glutathione yeast strains
Mutation breeding:
So far, the physical or chemical treatment methods for yeast strains produced by GSH generally include ultraviolet, X-ray, gamma ray, and nitroso guanidine.
Through a series of strains improvement and breeding, many strains with high GSH yield can be obtained.
Genetic engineering Breeding:
In the biosynthesis of GSH, the activity of GSH synthetase series is generally low,
and GSHⅰ is a rate-limiting enzyme whose activity is inhibited by GSH feedback.
In order to reduce the feedback inhibition of GSH on GSHⅰ, it is necessary to improve the activity of enzyme system or reduce the sensitivity of enzyme system to GSH.
Murata et al. successfully constructed recombinant Escherichia coli (E.coli) with strong GSH synthesis ability at an early stage,
screened a GSHⅰ desensitized mutant from E.coli B, cloned its GSHⅰ gene, and expressed it in E.coli BRC912. The ability of the obtained strains to synthesize GSH was greatly improved.
Optimization strategy of medium components
Medium composition is the key factor to obtain high GSH yield.
The interaction of different C-N sources in the medium composition is required to improve cell growth and obtain high intracellular GSH accumulation.
Liu et al. believed that glucose was the best carbon source for the growth of S.cerevisiae AT CC7754, and peptone was the best nitrogen source.
Wei Gong Yuan et al. studied the effects of different carbon and nitrogen sources on the fermentation of C. Utilis WSH02208 to produce GSH. The results showed that sucrose was conducive to promoting cell growth, and glucose had a great effect on the synthesis of GSH.
Single nitrogen source was significantly better than organic nitrogen source on cell growth and GSH production, and mixed inorganic nitrogen source had a significant effect on GSH production.
In addition to the interaction between C-N sources, other components,
such as salts and vitamins, are needed to determine optimal process conditions.
Amino acids are precursors of GSH biosynthesis.
Their addition affects cell growth and intracellular synthesis of GSH.
The biosynthesis of GSH requires ATP and three precursor amino acids, namely glutamic acid (Glu), cysteine (Cys), and glycine (Gly).
When there is no supplementation in the medium,
intracellular concentrations are maintained at their physiological levels and GSH synthesis may be inhibited.
Optimization of fermentation conditions and process control
Fermentation conditions optimization:
Environmental conditions are also important factors in the production of glutathione by fermentation,
and the environmental parameters that affect the content of GSH in cells mainly include temperature, dissolved oxygen, pH, etc.
Temperature directly affects the activity of various enzymes, metabolic reaction rate and the growth of bacteria in the metabolic process.
For example, Wei Gongyuan et al. have shown that higher temperature can reduce the inhibition of substrate on cell growth and is conducive to cell growth, while low temperature is more conducive to glutathione biosynthesis.
The pH in fermentation solution has an important influence on the dissociation state of nutrients,
enzyme activity and metabolic rate, so pH is also a key factor restricting the production of GSH.
The results showed that the total glutathione production the highest when pH value controlled at 5.5.
Glucose-adding strategy and fermentation process control:
The production of GSH is closely related to the consumption of glucose in fermentation broth. The study found that only when the glucose almost exhausted and the growth stopped, the large amount of GSH began to synthesized in the cell;
And since GSH is an intracellular product, it is necessary to increase the number of cells in the fermentation process to increase the total production of GSH while improving the ability of producing strains to synthesize GSH intracellular.
In order to achieve this goal, the mass concentration of the substrate must increased.
However, too high glucose concentration will produce Crabtree effect, inhibiting the growth of cells and greatly reducing the yield of target products,
so the late limited flow of glucose generally adopted to eliminate substrate inhibition.
At present, the most widely used glucose streaming strategies are:
Constant speed flow, exponential flow, feedback control flow.
Li Yin et al. compared the effects of constant rate flow addition, artificial feedback controlled flow addition and exponential flow addition on the synthesis of GSH in high density culture of recombinant E. coli, and found that exponential flow addition was the most ideal.
Stress response strategies to increase glutathione production
The study found that stress responses in yeast cells can triggered by the use of different strategies,
either individually or in concert, resulting in a range of defenses by the microbes that stimulate the production of GSH.
From a technical point of view, it is useful to understand the causes and effects of various types of forced degradation to establish process parameters and improve fermentation performance during the production of target compounds.
Different stress response induction strategies for saccharomyces cerevisiae include osmotic stress, MF application, high pressure, and the addition of carbon sources and GSH precursor amino acids in the feed batch process.
The downstream process of GSH extraction and determination
Sudiyani et al. use hot water, ethanol, phosphate, and H3PO4 for downstream processes, including extraction and concentration of GSH from yeast, followed by membrane separation.
The combination of ultrafiltration and nanofiltration is a promising method to concentrate and purify glutathione from yeast extracts.
Over the years, the techniques used to determine GSH and GSSG have evolved: spectrophotometry, fluorometry, chromatography and mass spectrometry.
There are several optimized rapid protocols available for GSH and GSSG analysis in biological samples with high sensitivity and accuracy:
UPLC-MS/MS, HPLC-UV-QTOF-MS, automatic flow based on regional jet and fluorescence detection, and real-time GSH monitoring ratio nanosensors.
Microbial metabolism used to convert low-priced nutrients into glutathione,
and the microorganisms used in fermentation easy to culture, cheap and easy to obtain nutrients, and the operation process simple.
The selection of high-yielding strains, mutagenesis breeding and genetic engineering breeding of wild strains,
and the fermentation production process in GSH are the research hotspots and breakthrough points of fermentation.
Further experimental studies and process optimization needed to improve glutathione production and reduce production costs, laying the foundation for industrial production.
References:
- [1] Liu Yifeng, Cao Xin-Zhi, Glutathione, a novel food additive, Journal of Fuzhou University (Natural Science Edition), 2002, 30, 714-721.
- [2] Chen Jian, Wei Gongyuan, Li Yin, Du Guocheng, Glutathione production by microbial fermentation, Journal of Wuxi University of Light Industry, 2004, 23(5): 104-110.
- [3] Zhang Xing, Cui Xiangwei, Li Zonglin, et al. Enzymatic production of glutathione based on energy recycling system. Journal of East China University of Science and Technology (Natural Science Edition), 2020, 46(5): 688-693.
- [4] Liao X Y, Shen W, Chen J, et al. Improved glutathione production by gene expression in Escherichia coli. Letters in Applied Microbiology, 2006, 43(2): 211-214.
- [5] Xu R Y, Wang D H, Wang C L, et al. Improved S⁃adenosylmethionine and glutathione biosynthesis by heterologous expression of an ATP6 gene in Candida utilis. Journal of Basic Microbiology, 2018, 58(10): 875-882.
- [6] Jiang Qiuqi. Improvement of glutathione synthesis efficiency by modification of Pichia pastoris. Master’s thesis. Wuxi: Jiangnan University, 2020.
- [7] Malairuang K, Krajang M, Sukna J, Rattanapradit K, Chamsart S, High cell density cultivation of Saccharomyces cerevisiae with intensive multiple sequential batches together with a novel technique of fed-batch at cell level (FBC). Processes 2020, 8:1–26.
- [8] Kresnowati M, Ikhsan N, Nursa’adah R, Santoso N, Susanto Y, Evaluation of glutathione production method using Saccharomyces cerevisiae. IOP Conf Ser Mater Sci Eng, 2019, 543:012004.
- [9] Chen H, Cao X, Zhu N, Jiang L, Zhang X, He Q, Wei P, A stepwise control strategy for glutathione synthesis in Saccharomyces cerevisiae based on oxidative stress and energy metabolism. World J Microbiol Biotechnol, 2020, 36.
- [10] Murata K, Kimura A. Some properties of glutathione biosynthesis deficient mutants of Escherichia coli B. J Gen Microbiol, 1982, 128: 1047 – 1052.
- [11] Kobayashi J, Sasaki D, Bamba T, Hasunuma T, Kondo A, Sustainable production of glutathione from lignocellulose-derived sugars using engineered Saccharomyces cerevisiae. Appl Microbiol Biotechnol, 2019, 103:1243–1254.
- [12] Liu CH, Hwang CF, Liao CC, Medium optimization for glutathione production by Saccharomyces cerevisiae. Process Biochem, 1999, 34:17–23.
- [13] Wei Gongyuan, Li Yin, Tu Guocheng, et al. Nutritional and environmental conditions of biosynthesis of glutathione by Candida utilis. Chinese Journal of Applied & Environmental Biology, 2003, 9(6): 643-644.
- [14] Lorenz E, Schmacht M, Stahl U, Senz M, Enhanced incorporation yield of cysteine for glutathione overproduction by fed- batch fermentation of Saccharomyces cerevisiae. J Biotechnol, 2015, 216:131–139.
- [15] Liu H, Lin JP, Cen PL, Pan YJ, Co-production of S-adenosyl- l-methionine and glutathione from spent brewer’s yeast cells. Process Biochem, 2004, 39:1993–1997.
- [16] Wei Gongyuan, Li Yin, Chen Jian, et al. Effects of dissolved oxygen and pH on glutathione production by batch fermentation of Candida prion producing. Chinese Journal of Biological Engineering, 2003, 19(6): 734-739.
- [17] Li Yin, Chen Jian, Lun Shiyi. Glutathione produced by high density culture of engineered bacteria. Chinese Journal of Pharmaceutical Industry, 1999, 30(1): 1-4.
- [18] Bleoanca I, Bahrim G, Overview on brewing yeast stress factors. Rom Biotechnol Lett, 2013, 18:8559–8572
- [19] Kresnowati M, Ikhsan N, Nursa’adah R, Santoso N, Susanto Y, Evaluation of glutathione production method using Saccharomyces cerevisiae. IOP Conf Ser Mater Sci Eng, 2019, 543:012004.
- [20] Santos L O, Silva P G P, Lemos Junior W J F, de Oliveira V S, Anschau A, Glutathione production by Saccharomyces cerevisiae: current state and perspectives, Applied Microbiology and Biotechnology, 2022, 106: 1879–1894.
- [21] Sudiyani Y, Faizal FA, Muryanto, Firmansyah I, Setiawan AAR, Glutathione from Saccharomyces cerevisiae as by-product of second-generation bioethanol from oil palm of empty fruit bunch fiber. In: Usman Safitri N (ed) IOP Conference Series: Materials Science and Engineering. Institute of Physics Publishing, 2019.
- [22] Wang Y, Xiao T, Zhang Z, Feng X, Extraction and concentration of glutathione from yeast by membranes. Can J Chem Eng. 2021.
- [23] Giustarini D, Dalle-Donne I, Colombo R, Milzani A, Rossi R, An improved HPLC measurement for GSH and GSSG in human blood. Free Radic Biol Med, 2003, 35:1365–1372.
- [24] Hakuna L, Doughan B, Escobedo JO, Strongin RM, A simple assay for glutathione in whole blood. Analyst, 2015, 140: 3339–3342.
- [25] Nolin TD, McMenamin ME, Himmelfarb J, Simultaneous determination of total homocysteine, cysteine, cysteinylglycine, and glutathione in human plasma by high-performance liquid chromatography: application to studies of oxidative stress. J Chromatogr B Anal Technol Biomed Life Sci, 2007, 852:554–561.
- [26] Bravo-Veyrat S, Hopfgartner G, High-throughput liquid chromatography differential mobility spectrometry mass spectrometry for bioanalysis: determination of reduced and oxidized form of glutathione in human blood. Anal Bioanal Chem, 2018, 410:7153–7161.
- [27] Herzog K, Ijlst L, van Cruchten AG, van Roermund CWT, Kulik W, Wanders RJA, Waterham HR, An UPLC-MS/MS assay to measure glutathione as marker for oxidative stress in cultured cells. Metabolites, 2019, 9.
- [28] Sun X, Berger RS, Heinrich P, Marchiq I, Pouyssegur J, Renner K, Oefner PJ, Dettmer K, Optimized protocol for the in situ derivatization of glutathione with n-ethylmaleimide in cultured cells and the simultaneous determination of glutathione/glutathione disulfide ratio by HPLC-UV-QTOF-MS. Metabolites, 2020, 10: 1–15.
- [29] Tsiasioti A, Tzanavaras PD, Determination of glutathione and glutathione disulfide using zone fluidics and fluorimetric detection. Talanta, 2021, 222.
- [30] Ahmad M, Anjum NA, Asif A, Ahmad A, Real-time monitoring of glutathione in living cells using genetically encoded FRET- based ratiometric nanosensor. Sci Rep, 2020, 10.