Individuals considering taking NMN supplements may wonder about the best way to administer them. While many people overlook this detail, the route Nicotinamide Mononucleotide takes into the body can ultimately determine its benefits to the user.
A proper delivery system will ensure maximum bioavailability, i.e. the fraction of the ingested compound that reaches the bloodstream in an active form. The effectiveness of a supplement depends largely on this bioavailability.
Most NMN delivery systems are not supported by clinical studies, given that delivery systems work differently for each compound.
Therefore, capsules are the only Nicotinamide Mononucleotide delivery system that has been repeatedly tested in humans. However, this does not necessarily mean that the capsule is a top-notch delivery system.
Therefore, the potential bioavailability in humans of each of the most common NMN delivery systems currently on the market will be discussed.
The first type: NMN capsules

Human studies have almost exclusively used encapsulated NMN supplements. In many of these studies, Nicotinamide Mononucleotide has been shown to increase blood NAD+ levels, suggesting that Nicotinamide Mononucleotide has strong bioavailability.
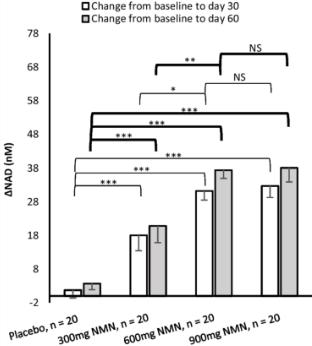
Additionally, one study showed that while 600 mg of encapsulated NMN increased blood NAD+ levels, 900 mg did not further increase NAD+ levels.
This stabilization of blood NAD+ levels over this dose range suggests that there may be a limit to the extent to which NAD+ levels can be increased using this delivery system.
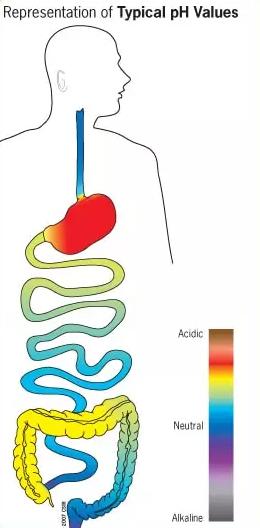
In some cases, special capsules called enteric-coated capsules can increase the bioavailability of compounds by taking advantage of the different pH levels of our gastrointestinal (GI) tract.
The pH of our stomach is very acidic, allowing it to destabilize many compounds. However, pH-sensitive enteric-coated capsules protect their contents by bypassing destruction in the stomach and dissolving in the intestines.
In this way, enteric-coated capsules may improve the bioavailability of Nicotinamide Mononucleotide, but this has not been extensively tested.
The second type: NMN powder
Powdered compounds are often placed in capsules, which have the advantage of hiding unpleasant tastes and preventing oral absorption.
However, some people may prefer to dissolve powdered supplements in water to avoid swallowing pills. Considering that NMN remains stable when dissolved in water, this may be a viable option for Nicotinamide Mononucleotide.
Additionally, many studies have shown that when NMN is put into the drinking water of mice, it increases their blood NAD+ levels, indicating its bioavailability.
Additionally, one study showed that Nicotinamide Mononucleotide does not degrade significantly in simulated stomach acid, which has a pH similar to our own.
Therefore, enteric-coated capsules may be unnecessary when it comes to Nicotinamide Mononucleotide bioavailability. More human studies are needed to determine whether the capsule form of NMN is more bioavailable than the powder form dissolved in water.
The third type: NMN suppository
NMN suppositories are similar to NMN capsules, placing NMN powder into a durable material. However, suppositories are larger and can store more Nicotinamide Mononucleotide.
Suppositories also bypass the stomach, so their contents may be less susceptible to degradation. However, whether Nicotinamide Mononucleotide suppository delivery systems make NMN more bioavailable than encapsulated NMN has not been tested. Additionally, many people may find suppositories uncomfortable and difficult to administer regularly.
Type 4: NMN oral spray and sublingual NMN
With Nicotinamide Mononucleotide Oral Spray, NMN is designed to be absorbed through the mouth, bypassing the gastrointestinal tract and allowing rapid absorption into the bloodstream.
Additionally, oral sprays often contain liposomal Nicotinamide Mononucleotide rather than the usual microcrystalline NMN.
Liposomes are bubble-like structures made from natural fats that mimic the structure our bodies use to transport various molecules. For this reason, liposomal NMN is considered to be more bioavailable than crystalline Nicotinamide Mononucleotide.
Other Nicotinamide Mononucleotide delivery systems similar to oral sprays include sublingual gels, tablets, and powders.
These delivery systems may be better at sustaining NMN absorption than sprays, as Nicotinamide Mononucleotide may remain in the mouth longer.
Additionally, the floor of the mouth beneath the tongue may be more permeable to Nicotinamide Mononucleotide than other parts of the mouth, making it more bioavailable.
However, it is unknown whether Nicotinamide Mononucleotide absorbed through the mouth has similar bioavailability to NMN absorbed through the gastrointestinal tract.
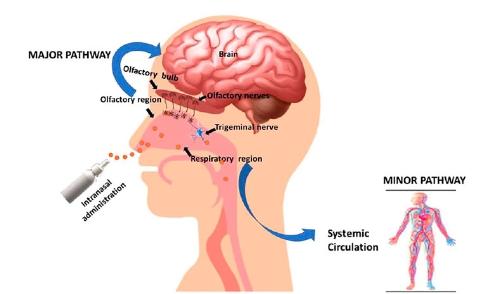
The fifth type: nasal spray
The intranasal route of administration of compounds has the advantage of bypassing the gastrointestinal tract and blood-brain barrier (BBB).
The blood-brain barrier prevents potentially harmful molecules in the peripheral blood from entering the brain.
Therefore, supplements and medications that cannot cross the blood-brain barrier cannot reach the brain when taken orally. However, studies in mice suggest that NMN crosses the BBB, so intranasal administration may not be necessary.
Additionally, the nasal cavity can degrade compounds, and it’s unclear how effectively Nicotinamide Mononucleotide nasal spray increases NAD+ levels in the brain or blood.
Type 6: NMN for external use on skin
Topical application of NMN involves dissolving Nicotinamide Mononucleotide in an oily solution and rubbing it into the skin.
One study showed that topical NMN reduced inflammation and skin damage in a mouse model of eczema. Another study showed that Nicotinamide Mononucleotide increased NAD+ levels in skin cells grown in lab dishes.
Therefore, topical Nicotinamide Mononucleotide may increase NAD+ levels in human skin. However, topical NMN may not significantly increase blood NAD+ levels.
Additionally, using other delivery systems to increase NAD+ levels in the blood may also increase NAD+ levels in the skin.
Type Seven: Top NMN Delivery System
Because most human studies have been performed only on microcrystalline-encapsulated NMN supplements, determining whether other delivery systems are comparable must be based primarily on animal studies.
Meanwhile, a Japanese study showed that injecting Nicotinamide Mononucleotide into healthy individuals increased blood NAD+ levels by 20% and reduced blood lipid levels.
Because the injection bypasses all of the body’s natural barriers, injecting Nicotinamide Mononucleotide directly into the bloodstream makes it nearly 100% bioavailable.
Caveats with injecting Nicotinamide Mononucleotide are the invasiveness and safety issues involved with piercing the skin with a needle. Additionally, there is a lack of injectable NMN products on the market.
In most rodent studies showing that NMN supplementation increases blood NAD+ levels, researchers either injected NMN into drinking water, mixed Nicotinamide Mononucleotide into food, or force-fed (oral gavage) NMN.
Except for injection, these administration methods are similar to drinking Nicotinamide Mononucleotide dissolved in water or other liquids.
Therefore, powdered Nicotinamide Mononucleotide dissolved in liquid or food may have similar effects to NMN capsules.
Nonetheless, since enteric-coated capsules can improve bioavailability, they can be considered the top NMN delivery system. More comparative studies of Nicotinamide Mononucleotide delivery systems will make this clearer.
Journal Reference
- Formica, M. L., Real, D. A., Picchio, M. L., Catlin, E., Donnelly, R. F., & Paredes, A. J. (2022). On a highway to the brain: A review on nose-to-brain drug delivery using nanoparticles. Applied Materials Today, 29, 101631.
- Gonçalves, R. F., Martins, J. T., Duarte, C. M., Vicente, A. A., & Pinheiro, A. C. (2018). Advances in nutraceutical delivery systems: From formulation design for bioavailability enhancement to efficacy and safety evaluation. Trends in Food Science & Technology, 78, 270-291.
- Liu, P., Chen, G., & Zhang, J. (2022). A Review of Liposomes as a Drug Delivery System: Current Status of Approved Products, Regulatory Environments, and Future Perspectives. Molecules, 27(4).
- Tang, Z., Bao, P., Ling, X., Qiu, Z., Zhang, B., & Hao, T. (2023). In vitro digestion under simulated saliva, gastric and small intestinal conditions and fermentation of nicotinamide mononucleotide, and its effects on the gut microbiota. Food Research International, 113779.
- Shade, C. (2020). The Science Behind NMN–A Stable, Reliable NAD+Activator and Anti-Aging Molecule. Integrative Medicine: A Clinician’s Journal, 19(1), 12-14.